Lumigan® Eye Drops 3mL (bimatoprost ophthalmic solution 0.03%)
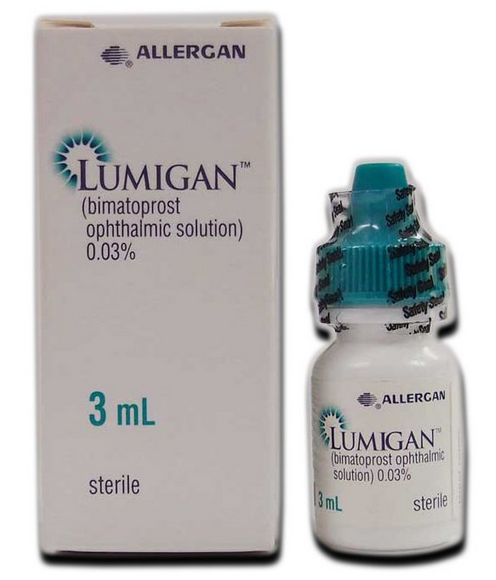
Lumigan® Ophthalmic Solution 0.03%
- Brand Name: Lumigan® 0.03% (3 mL)
- Composition: Bimatoprost ophthalmic solution
- Indications: To reduce intraocular pressure in glaucoma; Treatment of hypotrichosis of eyelashes (insufficient growth of eyelashes)
- Manufacturer: Allergan Pharmaceuticals
- Country of origin: Ireland
Lumigan® Cheap Price in US
Lumigan® Description
- Lumigan® for patients belongs to the drug group of prostamides . Prostamides have a number of applications in medicine, one of which is the expansion of the channels of outflow of intraocular fluid.
- Lumigan® thus copes with its task of reducing intraocular pressure, contributing to the outflow of excess intraocular fluid.
How does Lumigan® work?
The mechanism of action by which bimatoprost reduces intraocular pressure in humans is to increase the outflow of intraocular fluid through the trabecular mesh and increase the outflow from the uveoscleral parts of the eye. Reduction of intraocular pressure begins approximately 4 hours after the first application. The maximum effect is achieved within about 8-12 hours. The duration of the effect is at least 24 hours.
When is it recommended to use Lumigan® drops?
The drug Lumigan® Bimatoprost is recommended for use in:
- hypotrichosis (insufficient growth) of eyelashes;
- excessive loss of eyelashes;
- reduction of elevated intraocular pressure in adult patients with chronic open-angle glaucoma and intraocular hypertension (as monotherapy or additional therapy to beta-blockers).
Composition of Lumigan®
- active substance: bimatoprost;
- 1 ml contains 0.3 mg of bimatoprost;
- Excipients: benzalkonium chloride, sodium phosphate, heptahydrate; citric acid monohydrate, sodium chloride, hydrochloric acid diluted or sodium hydroxide purified water.
Dosage form
- Eye drops, solution.
- Basic physico-chemical properties: transparent, almost colorless solution.
Lumigan® Pharmacological Group
- Means used in ophthalmology.
- Anti-glaucoma drugs and myotic agents.
- Prostaglandin analogues.
Method of application of Lumigan® eye drops
- Lumigan® eye drops are used 1 time a day. Wash your hands before use. Tilt your head back and slightly pull the lower eyelid down to create a space between the eyelid and the eye.
- Take your eyes off the pipette and drop one drop into the space between the eyelid and the eye and close your eyes. Keep your eyes closed for about a minute to prevent drops from leaking out.
- Before using Lumigan®, it is necessary to remove contact lenses, since the drug contains preservatives that can be absorbed by the lenses. The lenses can be put on 15 minutes after using the drops.
- Do not touch any surfaces (eyes or hands) with the pipette to avoid contamination of the pipette and the occurrence of eye infections.
How to use Lumigan® for eyelash growth?
- Lumigan® drops are applied to the upper eyelid along the roots of the eyelashes with a special applicator 1 time a day.
- The duration of the course is 3 months.
- In case of contact with other areas of the eye, it is immediately removed with a cotton pad.
Contraindications
Hypersensitivity to the active substance or excipients included in the preparation, including benzalkonium chloride.
Pregnancy and breast-feeding
- The consequences of using Lumigan® during pregnancy and lactation have not been properly studied. There is no data on whether Lumigan® is excreted together with breast milk. Therefore, it may be recommended to stop breastfeeding for the period of Lumigan® treatment.
- Lumigan® should be used during pregnancy only if absolutely necessary, when the expected benefit to the mother exceeds the potential risk to the fetus.
- The consequences of using Lumigan® in children have not been studied, therefore Lumigan® is not prescribed to children.
Lumigan® Storage Conditions
- Does not require special storage conditions. Do not freeze. The shelf life of the drug after the first opening of the dropper bottle is 28 days.
- Shelf life is 2 years.
- Do not use after the expiration date indicated on the package.
Lumigan® for eyelash growth
The manufacturer Lumigan® promises that the eyelashes will reach their maximum length in 16 weeks of using the product. On the forums, girls praise this product, our customers also tried Lumigan®, and were satisfied with it. The composition includes 0.03% bimatoprost, which actively affects the follicles and hairs. But we want to warn you: these are hormonal drops, so be careful with the product. If you notice itching or redness in the eyelid area, stop using it.
Expressive eyes with long and thick eyelashes can correct any flaw on the skin, because all eyes will not be directed at them. A good remedy for eyelash growth can help to radically change the length of your eyelashes in a few weeks, which will give the look languor and depth.
Immediately it is worth noting a small but significant detail: the length of the eyelashes and their density are genetically embedded. Do children have long and fluffy eyelashes, but adults don’t? This is because the external environment, poor nutrition, lack of vitamins, as well as improper care or its complete absence lead to weakening of the ciliary follicles. Therefore, the cilia become thinner, break, fall out, losing their density and natural length.
Lumigan® eyelash growth activators will help restore the genetically inherent length and fluffiness, but do not expect that your eyelashes will be like extensions, if it is not inherent in nature.
Lumigan® Side effects
During the clinical trial, adverse reactions occurred in approximately 38% of patients treated with Lumigan® eye drops, 0.1 mg/mL. The most common adverse reaction (in 29% of patients) was conjunctival hyperemia (mostly mild and non-inflammatory). Approximately 4% of patients stopped using the drug due to a side effect that occurred during the study.
During clinical studies of Lumigan® eye drops, 0.1 mg/mL, the following adverse reactions were detected. Most of them were from the side of the visual organs, had a mild form, and none were severe. Possible side effects:
- dry eyes;
- visual impairment;
- burning sensation in the eyes;
- foreign body sensation;
- darkening of eyelashes;
- feeling of irritation;
- conjunctival edema;
- conjunctivitis;
- eye discharge;
- pigmentation of the iris.
Lumigan® bimatoprost solution can lead to a gradual change in the color of the eyes, eyelids and eyelashes. Such changes are very minor and may be unnoticeable for several years. Usually, the changes manifest themselves in the eye that is being treated, which can lead to cosmetic eye differences.
Interaction of Lumigan® with other drugs and other types of interactions
The interaction study was not conducted.
No interaction is expected in humans due to the fact that the systemic concentrations of bimatoprost are extremely low (less than 0.2 mg / ml) https://bimatoprost-3.com in the body after the application of bimatoprost solution at a dose of 0.3 mg / ml in the form of eye drops.
Preclinical studies have shown that bimatoprost is metabolized in the body using any of the numerous enzymes and metabolic pathways and does not affect the liver enzymes involved in the metabolism of drugs.
During clinical studies, bimatoprost solution in the form of eye drops was used simultaneously with several different ophthalmic beta-blockers (timolol 0.5%) without signs of interaction.
The simultaneous use of Lumigan® and glaucoma drugs, except topical beta-blockers, has not been studied with additional glaucoma therapy.